Studies have shown that fibrin sheath formation occurs in almost all types of catheter accesses. Fibrin sheath is the most common cause of catheter dysfunction; in addition, it can lead to a series of complications such as secondary infection, thrombosis, catheter removal, and pulmonary embolism. Therefore, early prevention, early detection, and early intervention to avoid severe complications caused by fibrin sheath formation and prolong catheter use have become hot topics in clinical practice.
1. What is a Fibrin Sheath?
.png)
(The image is sourced from the Internet)
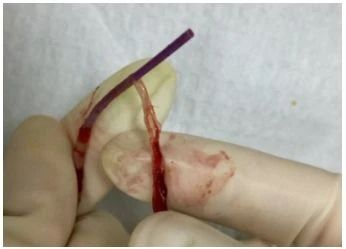
(The image is sourced from the Internet)
A fibrin sheath is a membranous structure composed of endothelial cells, smooth muscle cells, collagen, and fibrous connective tissue. When wrapping around the catheter surface, it can cause thrombosis, catheter dysfunction, secondary infection, adhesion to the vascular wall leading to difficult catheter removal, and even pulmonary embolism due to shedding after removal. This membranous covering has been successively described by names such as fibrin sleeve, fibrin sheath, fibrinous thrombus sleeve, and cuff.
2. Pathogenesis of Fibrin Sheath?
Currently, domestic literature on intravenous therapy points out two viewpoints regarding the formation of catheter-induced fibrin sheath.
The first viewpoint holds that fibrin sheath is formed by the deposition of proteins in blood on the catheter surface followed by organization of secondary thrombosis, and its essence is an organized thrombus.
The second viewpoint suggests that fibrin sheath formation is a biological response of smooth muscle cells and endothelial cells in the venous wall to catheter components and related thrombi, serving as a protective response of the venous wall to the foreign body (catheter) and a process of the body's self-regulatory repair.
Studies have shown that this sheath contains fibrin components only in the early stage, but not in the mature stage. Fibrin-containing thrombi are prone to fragmentation and can be dissolved by thrombolytic agents, while mature sheaths, being dense fibrous connective tissue, cannot be dissolved by thrombolytic agents. Fibrin sheath is considered a structurally stable tissue; some scholars observed the residual fibrin sheath in blood vessels after catheter removal and found that the sheath remained firmly attached to the venous wall 10 months after removal.
3. Formation Process of Fibrin Sheath?
Endothelial loss occurs at the venous puncture site, and a thrombus forms between the puncture site and the catheter, forming a "thrombotic bridge". Gradually, smooth muscle cells migrate from the venous wall to the catheter side through this thrombotic bridge. In addition, venous wall endothelial cells also crawl along the thrombotic bridge to the catheter surface, gradually forming a sheath dominated by smooth muscle cells and collagen, covered by endothelial cells, and growing distally along the catheter wall.
4. Causes of Fibrin Sheath Formation?
Relevant data indicate that fibrin sheath formation is associated with catheter-related thrombosis.
The three major factors for thrombosis are:
1.Vascular endothelial injury
2.Blood stasis
3.Hypercoagulable state of the body
These three factors are the main causes of thrombosis. Studies have confirmed that catheter insertion can directly cause vascular endothelial injury; in addition, unskilled puncture techniques, repeated punctures, and improper operations can also lead to vascular endothelial damage. During catheter indwelling, strenuous exercise can cause friction between the catheter and the vascular intima, resulting in mechanical stimulation of the vascular wall and inducing thrombosis. Moreover, reduced activity, insufficient water intake in elderly patients after catheterization, and the occupation of vascular volume by the indwelling catheter leading to slow blood return all contribute to thrombosis. Patients in a hypercoagulable state are at high risk of thrombosis. The interaction of these three factors is the main cause of catheter-related thrombosis and is considered by many experts and scholars as the basis for fibrin sheath formation.
5. Time of Fibrin Sheath Formation?
Studies suggest that fibrin sheath formation is a dynamic process. No fibrin sheath forms within 6 hours after catheter insertion; it begins to form after 12 hours of indwelling. Fibrin sheaths formed 1-2 weeks after catheter insertion have no clinical symptoms, while later-formed sheaths can cause catheter dysfunction and even difficulty in catheter removal.
The currently accepted view is that fibrin sheath starts to form at the contact point between the catheter and the venous wall 24 hours after catheterization, then extends along the catheter wall, gradually "covering" the entire wall, and takes approximately 5-7 days to reach the full length of the catheter wall. Animal experiments have confirmed that the incidence of fibrin sheath reaches 100% one week after catheterization. Clinical angiography has confirmed a 76% incidence of fibrin sheath. Additionally, clinical observation of color Doppler ultrasound in temporary hemodialysis catheters from insertion to one month after removal found that almost all catheters began to form mural thrombus at the venous entry site one day after insertion, marking the onset of fibrin sheath formation.
6. Manifestations of Catheter-Related Fibrin Sheath?
Fibrin sheath wraps around the catheter surface and may extend along the catheter to form a valve-like structure. A characteristic manifestation is that the catheter is patent when pushing fluid, but the valve is sucked by negative pressure to block the catheter tip during aspiration, resulting in poor aspiration and catheter dysfunction. Common clinical manifestations of catheter dysfunction caused by fibrin sheath include puncture site exudation, decreased infusion rate, catheter occlusion, no blood return on aspiration, and difficulty in catheter removal.
7. Imaging Diagnosis of Fibrin Sheath?
Currently, there is no gold standard for the imaging diagnosis of fibrin sheath. Studies suggest that the characteristic sonographic feature of fibrin sheath on color ultrasound is thickening of the catheter wall; however, it is also noted that ultrasound has limitations, as visualization is unsatisfactory in patients with thick adipose tissue.
8. Management of Catheter Fibrin Sheath?
Thrombolytic therapy with drugs such as urokinase, streptokinase, and alteplase can be used. Pumping urokinase through the catheter to contact and dissolve fibrin and red blood cells can effectively prevent the formation of thrombus and fibrin sheath.
In-situ replacement of the dysfunctional or thrombus-occluded catheter with a new one.
A stripping device can be inserted into the vein to pull off the fibrin sheath from top to bottom, or a stripping device can be inserted through the original catheter to rupture and strip the fibrin sheath in situ. However, this may inevitably cause fragmented fibrin sheath to enter the bloodstream, with the potential risk of pulmonary embolism if it enters the pulmonary artery.
9. Prevention of Catheter Fibrin Sheath?
1.Standardized maintenance of the catheter after insertion is crucial for ensuring normal catheter use and long-term indwelling! Use vascular visualization technology to improve puncture success rates in patients with difficult venous access.
2.Select appropriate catheter size and insertion vessel based on the patient's vascular conditions and treatment needs.
3.The vortex generated by the push-pause-push pulsatile flushing technique can effectively flush attachments and drugs adhering to the inner catheter wall, and the positive pressure capping method not only reduces catheter occlusion but also causes catheter "shaking", effectively preventing fibrin formation.
4.Use a 10ml syringe to flush the fibrin tail or sheath at the catheter tip by increasing flushing frequency. Smaller syringes may cause catheter or vascular endothelial damage due to higher pressure, while larger syringes may not achieve effective flushing.
5.If blood sampling, blood transfusion, or infusion of other viscous fluids is performed through this catheter, manual flushing must be done first before connecting other fluids.
6.Regularly monitor the catheter infusion rate; promptly identify and properly address the cause if the rate decreases.
7.Regularly observe the puncture site for redness, induration, exudate, or other abnormalities, and provide timely local treatment if present.
8.Advise patients to wear loose-cuffed clothing, as tight cuffs can impede blood circulation and accelerate thrombosis.
9.Strengthen home education for outpatients, instructing them to closely monitor upper arm circumference, measure it once daily, and keep records.
10.Encourage the use of various non-pharmacological methods for thrombosis prevention, such as early limb movement, mild physical exercise, and adequate fluid intake.
References
[1] Li R Q, Jiang D Q, Tang Z M. Observation and nursing of PICC fibrin sheath formation in cancer patients [J]. Nursing and Rehabilitation Journal, 2013, 12(7): 681-682.
[2] Jia L Z, Lu Y, Ren X H. A case of difficult catheter removal and in-vivo catheter fragmentation caused by PICC-related fibrin sheath formation [J]. Chinese General Nursing, 2017, 15(19): 2428-2429.
[3] Zhou X Z, Zhong T, Qiu X M, et al. Clinical analysis of difficult removal of peripherally inserted central catheter due to fibrin sheath formation in children [J]. Chinese Journal of Vascular Surgery (Electronic Edition), 2018, 10(4): 283-286.
[4] Qiao A Z. Analysis of typical difficult cases of PICC [M]. Beijing: Science Press, 2018: 248-251.
[5] Cai Y W, Wang Y P, Tu Y W, et al. Nursing research on thrombosis and fibrin sheath formation in central venous catheters of hemodialysis patients with kidney disease [J]. Chinese Modern Doctor, 2017, 55(12): 153-156.
[6] Mei L. Causes and nursing countermeasures of difficult removal of peripherally inserted central catheter [J]. Health Care Guide, 2017, 16(3): 90.
[7] Jia L Z, Lu Y, Ren X H. A case of difficult catheter removal and in-vivo catheter fragmentation caused by PICC-related fibrin sheath formation [J]. Chinese General Nursing, 2017, 15(19): 2428-2429.
[8] Motin J, Fischer G, Evreux J. Importance of the subclavicular. Qilu Journal of Nursing, 2019, 25(1): 108.
[9] Cao L P, Yan W N. Research progress on the pathogenesis and recanalization methods of PICC catheter fibrin sheath-induced occlusion [J]. Chinese Nursing Research, 2017, 31(31): 3912-3914.
[10] Xu L Q. Analysis of related factors of central venous catheter infection during cardiothoracic surgery [J]. Medical Journal of Chinese People's Health, 2018, 30(16): 76-78.
[11] Tang W S, Liao H T, Liu D, et al. Research progress on detection, prevention, and treatment of venous indwelling catheter-related fibrin sheath [J]. Chinese Nursing Research, 2017, 31(21): 2575-2579.
[12] Infusion Nurses Society. Infusion therapy standards of practice [J]. J Infus Nurs, 2021, 41(1S): S113-S116.



.png)
