DISINFECTION OF NEEDLELESS CONNECTOR HUBS: CLINICAL EVICDENCE SYSTEMATIC REVIEW
Intravenous catheters and those related devices used to gain access to the veins for the purpose of infusing medications or solutions have evolved significantly over the past three decades. One if the more noticeable changes involves the way intravenous devices are accessed. Early concerns over needle safety for healthcare workers led to the creation of products that provide needle-free access.
While these products did eliminate the risk of accidental needle injury for the clinician, some needleless products raised new issues for the patient; namely, a noted increase in the occurrence of catheter associated bloodstream infections (CABSI) AND central line associated bloodstream infections (CLABSI). [1-3] Risk factors for infection include poor adherence to aseptic technique, needleless connector (NC) design variations, and inconsistent health care staff education and training.[1-3]
NC are used on virtually all intravascular devices in the USA ; they provide an easy access point for syringe or tubing attachment and have now become the central access point for all connections. Yet, despite providing some level of safety, concerns over infection related to NC contamination exist. Surface design, gaps around valve closure surface, segmented fluid pathway with dead space, differing internal mechanisms, clear or obscured visibility, variable blood reflux, clamping sequences, and different flushing instructions, depending on the type of NC, all play a part in the level of risk associated with the device. Before the advent of NC, clinicians had an intuitive understanding that prior to penetrating the septum with the needle the septum required disinfection. Current surface disinfection of NC is not necessarily intuitive. Initially, needless split septum access points used a blunt “ needle- looking” type cannula. As a result, the disinfection process remained intuitive.
Split septum access devices continue to be recommended as a lower risk option for needleless connection; hower, they have lost popularity because they require multiple parts and pieces for access and allow direct needle access through the septum /diaphragm leading many facilities to switch to luer access devices. With the changes to the access point using direct luer connection through the NC, the intuitive sense to disinfect the surface prior to access is lost; many clinicians fail to realize the consequences of this breech in aseptic technique[4-6]. colonization of catheter hubs and NC, with subsequent bacterial ingress into the catheter lumen, is considered the cause of 50% of postinsertion catheter-related infections [3-7]. Disinfection of the exposed surface of the NC is necessary to avoid contamination and subsequent intraluminal biofilm formation and protect patients from infection.Vast improvements have been made in the reduction of CLABSIs attributed to insertion procedures.
The results of the groundbreaking Keystone initiative demonstrated the effect of five measures, known as the Central Line Bundle, on the improvement of outcomes during insertion of central venous catheters[64-105]. Consistent application of the bundle, with compliance verified during the insertion procedure(checklist), has reduced insertion related CLABSI by more than 44% in the USA.[52] Even in institution where full compliance of the bundle exists, CLABISs are still occurring[108].Disinfection of the NC access site was not included in the insertion related central line bundle. The goal of any effective prevention program is zero CLABSIs. To reach the goal od zero, consideration must be given for the pathogenesis of catheter related infections and an investigation into current human factors of catheter management preventing achievement of this goal.
While many experts agree that application of the insertion bundle is one of the best ways to prevent insertion-related infection, the bundle dose not address NC, aseptic access, or any postinsertion catheter usage issues. A Pennsylvania study reported that 71.7% (468/653)of central line infection occurred five days or more after insertion and may have been directly related to use and care of intravascular devices [60,108-110].
Contamination of the catheter directly through the catheter hub has been confirmed through published studies. These studies found that bacteria identified on external hub surfaces were also present in biofilm sampled from random locations within the needless connector. Research performed at one institution revealed that patient skin flora was not the source of catheter related bloodstream infections in any of their cases; all infections in this study originated from the catheter hub.[6,113].
Infections later in the life of the catheter develop form improper catheter manipulation, failure to perform hand hygiene, inadequate time to clean NC, inadequate training, and poor access and exit site management[2,67,110,112]. Disinfection of a catheter hub prior to flushing or prior to the administration of medications is required for all aseptic access, yet in the Karchmer study, 31% of clinicians did not even attempt to disinfect, even when under active observation [1,64,88,114]. In a study by Lee the disinfection compliance by clinicians prior to NC access was measured at only 10%[115]. This common break in aseptic technique sets the stage for biofilm formation within NC and catheters and increases the potential for delayed infection of both central and peripheral catheters[14,60,68,112,116]. The results of the Pennsyvania Patient Safety Advisory Report and independent biofilm sampling of NC suggest that more attention is needed for aseptic access and maintenance practices[109].
Epic3 further state that disinfection methods used in combination with cleaning blood or other debris off the surface as disinfectants have limited ability to penetrate organic material[8]. The Association for Professionals in Infection Control (APIC) defines disinfection as a process to eliminate microorganisms accomplished with the use of liquid chemicals or pasteurizing; process works best by having proper contact time and dilution of disinfection agent[117]. Recommendations from the Centers for Disease Control, the Agency for Healthcare Research and Quality, the Society for Healthcare Epidemiology of America[10], and the Infusion Nurses Society[11,118] state that NC should be consistently and thoroughly disinfected using mechanical friction with 70% alcohol, alcoholic chlorhexidine, or povidone iodine prior to each access of an intravascular device and listed in evidence as a Category 1A.
The goal of this review is to access current literature related to disinfection of NC to establish recommendations that promote aseptic access, reducing infection risk of the patient.
The systematic review of there topics yielded a total of 433 papers and abstracts. After initial review 259 articles did not meet eligibility requirements and were removed. Included studies consisted of 140 publications dealing with disinfection/catheter hub/ NC contamination with 34 abstracts/posters. Of the studies 67 were graded according to the strength of the study.
A catheter is inserted into a vein or artery to provide a pathway for the administration of medications or solutions necessary to improve a patient’s health or condition. Because catheters provide an open conduit into the vasculature, a NC is attached, via luer threaded connection, to the integrated hub end of the catheter establishing a closed system. In a prospective controlled study by Rosenthal and maki and multicenter prospective cohort by Rangel Frausto et al., open systems compared to closed systems resulted in major reductions in catheter related infections[120]. NC used as a closed system must be weighed with consideration for potential negative factors of design features, poor aseptic practices, and lack of disinfection that all contribute to risk of infection[2,121,122].
Any puncture through the protective skin barrier creates a portal for bacteria to enter the body. Recognized routes of catheter contamination are classified as either extraluminal or intraluminal and include:
- Migration of microorganisms from the skin at the insertion site (considered the source in short term infections);
- Catheter hub contamination;
- Hematogenous seeding from another infection source in the body;
- Direct contamination from an infusate.[8,108,123].
After insertion of a catheter, introduction of microorganisms occurs primarily from two routes: the skin/insertion track or through the lumen of the catheter[15,124-127]. the greatest risk for contamination of the catheter after insertion is the access hub with 33-45%(402/900) contaminated in normal patient use[6,15,128-132] . In early studies by Sitges-Serra colonization of the catheter hub was considered the primary pathogenesis of catheter associated infection[15,113]. Linares and colleagues reported 14 episodes of sepsis (70% of total catheter related septic events) resulted from hub- related contamination[127,133].
Studies indicate that, during periods of nonuse, colony forming units(CFU) are present on access hubs in numbers ranging from 15 to 100CFU, representing quantities sufficient to cause contamination, biofilm formation, and potentially bacteremia if not sufficiently disinfected prior to access[3,5,12,99,110,111,134-143]. As demonstrated by multiple studies, infections are drastically lower or eliminated by disinfecting or covering the access hub with an antimicrobial cap[14,16-20,113,127,144-146].
Hub contamination plays an increasingly important role with infection risk the longer the catheter is in place. Intraluminal contamination and subsequent colonization become more prominent with longer dwell times[110,147]. Perez and associates found 59%(42/75) of one group of NC colonized with biofilm and Salzman found that 71%(20/28) of catheter related infections originated in the catheter hub presumably from contamination[15,21,22,148]. Clearly hub contamination is a causative element in catheter related infections and one that demonstrates the necessity for effective hub disinfection prior to access[110,113,127,133,144,149].
Disinfection points to gain access to intravenous or intravascular devices may include tubing side ports, direct catheter connections, stopcocks with needle free caps, NC of various types (split septum,mechanical valves, positive pressure valves, zero, or neutral connectors ), traditional silicon septum , or other forms of access integrated with the catheter or tubing. Any intravascular access point with a surface open to the environment requires disinfection prior to use, as it acts as the immediate portal of entry for intraluminal contaminants[23,99,113,127,133,144,150-152].
Needle free devices constitute more than 80% of access devices, are recommended by Centers for Disease Control for all tubing /catheter access, and are now more common than traditional covered septal access ports which allowed needless to pass easily through the silicone or rubber covered access[8,153]. Primary areas of focus for disinfection of access sites are the point where the sterile syringe or tubing contacts the site, as in the top septum surface, and the threads or side surfaces[7,143,154]. Manufactures are required to include instruction for device use and disinfection recommendations with each product to guide in the correct and safe usage of the NC.
Effective disinfection of a NC is influenced by several factors including: ability to clean the NC surface, the amount and position of grooves or gaps present, and the roughness or smoothness of the septum[1,3,7,69,150,154-156]. All NC consist of a septum, a fluid pathway and a mechanism for activation; the design ,space, volume, and human factors all affect how easy a product is to use and disinfect, and may also act as contributors to the potential risk of catheter associated bloodstream infection[3,69,150,155,157,158]. NC have gaps of differing widths between the septal seal and the housing which may allow ingress of microorganisms [7,23,99,143]. Adequate cleaning or need for additional cleaning of the septal access site may be based on the specific design features of the individual NC[2,4,5,7,70,111,129,153,159-161].
Recommendations from both the Center of Disease Control and the Infusion Nurses Society state clinician should minimize contamination risk by disinfection the access ports of the add-on device using friction with an appropriate disinfectant(70% alcohol, chlorhexidine, povidone iodine,and iodophors) prior to any access [8,11,24,167,168]. The 70% isopropyl alcohol wipe is most commonly used to disinfect the access surface of NC and has been proven effective or ineffective at disinfection times from 5 seconds to 60 seconds[14,19,24-27,131,151,169-177].
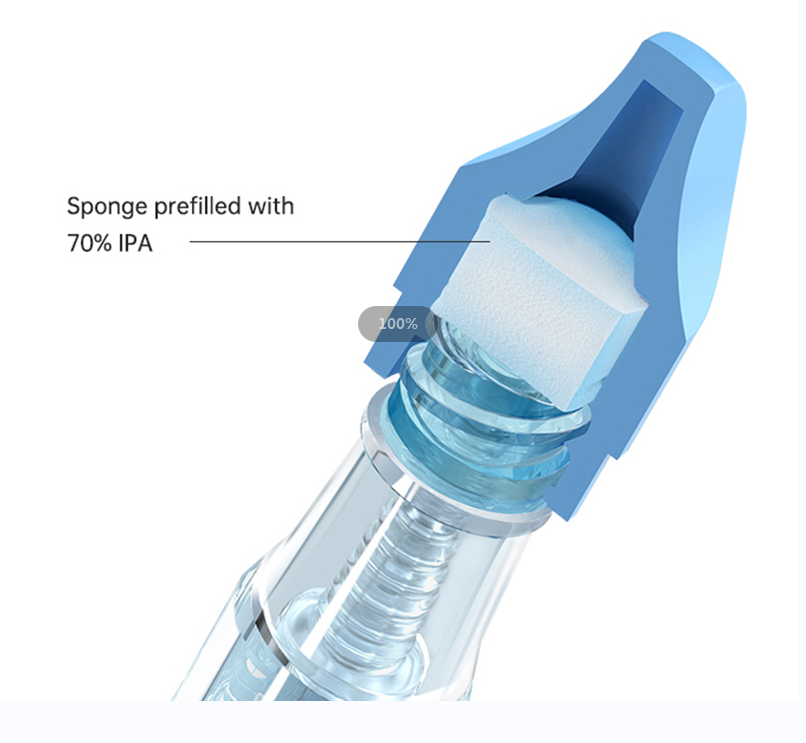
In a randomized prospective trial by Pittiruti,46 catheters received a 70% alcohol port protector with no detected CLABSIs over 707 catheters days, colonization in two catheters and no contaminated blood culture[18]. Of note the Pittiruti study resulted in reductions of CLABIs in the port protector/disinfection cap group and the control group, with improvements attributed to both the disinfection caps and educational efforts. These disinfection caps applied and left in place provide active mechanical friction along with longer contact time creating a physical and chemical barrier between the lumen and the environment[26].

In another retrospective study, Schears noted a pre-disinfection cap CLABSI rate of 1.682/1000 catheter days after implementing disinfection caps, representing a statistically significant 61% reduction in CLABSI[32]. in Wright et al.’s study at North Shore University Health System, a four University Hospital system, the intervention with 70% alcohol disinfection caps reported CLABSI rates declining from 1.42/1000 catheter days (16/11540) to 0.69/1000 (13/18972) with a 95% confidence interval, based on 799 enrolled patients, representing a statistically significant decrease[26].
Another engineered solution for hub cleaning involves a 70% alcohol foam cap designed for use ad an access site cleansing cap for single use, then discard. The Holroyd in vitro study at University of Florida compared the single use of this cleansing cap with 70% alcohol to traditional 70% alcohol wipes[151]. When the cleansing cap was used on stopcocks Holroyd found contamination and increased CFU. This study found 70% alcohol wipes and this alcohol cleansing cap were both effective on the surface of NC and catheter hubs[151]. Other groups also used this single use cleansing cap in combination with 70% alcohol disinfecting caps designed to be left in place until the next access.
Since the advent of NC as access hubs for the administration of medications or fluids, there has been a need to verify compliance with disinfection practices prior to access, During the period of needle usage for catheter access, nurses and doctors intuitively knew the necessity of disinfecting the access septum prior to inserting a needle. With NC, these questions arise: is disinfection always performed prior to access? Is disinfection performed in an effective manner? Do clinicians fully understand the consequences of not performing disinfection?
Catheter associated infections are a significant safety issue, and contamination caused by lack of aseptic technique is preventable. Once contamination occurs, bacteria attach to the inner lumen of the catheter, begin to grow and form biofilm, making successful eradication extremely difficult[[6,28-30,113,127,133,140-142,144,195]. Joint Commission now require hospitals in the USA to protect patients by having a standard and measurable protocol for hub/-access site disinfection[61,62,196]. Measurement of compliance with hub disinfection of the action unless disinfection caps/ports are used on all NC hubs are a form of verification. Passive disinfection through hub protectors/disinfection caps have differing designs and colors, leading to easy recognition and validation of compliance with usage. Reimbursement structures in the USA that now promote pay for performance and penalize poor outcomes will assist in driving these passive safety strategies that aid in monitoring and improving compliance with disinfection.
Even with the access of the Central Line Bundle on CLABSI reductions,a majority of hospitals remain well above zero for infections. Access and maintenance activities with the catheters maybe to blame. When CLABSIs occur well after the 96 hour mark, contamination of the catheter through the NC is likely the culprit. From the evidence presented, NC and catheter hubs are a primary source of bacterial contamination, and subsequent transmission of contamination into the catheter lumen[6,30,113,127,133,142,144]. More and more studies are demonstrating lack of compliance with hub disinfection despite educational initiatives and better disinfection agents. Disinfection methods that incorporate prolonged duration of contact with an antiseptic agent to significantly decrease the level of surface bacteria present may provide a solution to the problem of hub contamination and variation in NC designs.
In an evaluation of 5877 physicians, nurses and technicians, Jardim et al. Documented compliance with hub disinfection 38.7% of the time, leaving more than 60% of accesses without disinfection, leading to possible contamination and biofilm growth[92]. Platace et al. Evaluated clinician hands during invasive procedures demonstrating 100% of the 48 nurses sampled exceeded acceptable levels of microorganisms, with the potential to contaminate and cause bloodstream infection.
Various studies provide statements regarding conformity or lack of conformity concerning disinfection practices, attributing noncompliance to a lack of universal protocols, excessive workloads(e.g., when clinicians become busy, they are less likely to comply), or just forgetting to bring alcohol wipes to the bedside.[54,64,72,89-93,188].
The Smith study on behavioral intention indicated a negative correlated between performing optional disinfection with increasing age of clinicians and more years of experience[91]. Clearly there are human factors working against disinfection of hubs prior to access requiring engineered solutions such as passive disinfecting cap strips hanging on intravenous pump poles, supply dispensers of alcohol wipes to ensure greater,even 100% compliance with disinfection each and every time.[91,170].
Aseptic technique is the foundation for safe delivery of intravenous medications and solutions. More and more studies reveal lack of compliance with disinfection of access ports prior to and after access, despite educational initiatives, and better disinfection agents[1,26,27,38,54,57,64,69,72,74,78,8388-93,188,198-205]. Rather than creating devices such as the ultraviolet C port to eradicate contamination within the hub, the goal should be to eliminate surface pathogens before entering the NC or catheter. Passive disinfection caps reduce guess work, provide clinicians with a point of use solution, and reduce contamination.